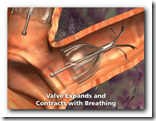
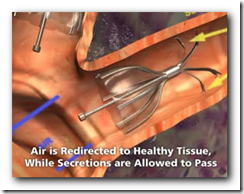
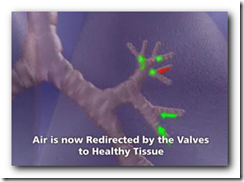
This technology will be used to increase air flow and re-direct to portions of the lungs that are not damaged. It will also expand and shrink with airflow. It somewhat appears though that there might be a larger crowd in need of the device though with all the lung related diseases we have today though. Biopsy forceps can remove the device if necessary as well. BD
Spiration said Friday that the U.S. Food and Drug Administration approved the use of its technology to control prolonged air leaks of the lung.
The Redmond company said the agency granted a rare Humanitarian Device Exemption from a more rigorous process.
It's the first FDA approval for a bronchial valve implant, the company said. The device consists of valves that can be inserted inside the lung's airways through a catheter.
The FDA allows some devices to be marketed for humanitarian use if they treat a condition that affects less than 4,000 U.S. patients per year.
Business & Technology | Spiration device gets FDA approval | Seattle Times Newspaper




0 comments :
Post a Comment